Understanding trait inheritance is crucial for unraveling the complexities of life’s blueprint and how it shapes the world around us. It provides insights into the transmission of characteristics from one generation to the next, shedding light on the mechanisms that underpin biological diversity and evolution.
In a groundbreaking study featured in the journal Nature, researchers have unveiled the hidden secrets of how our DNA replication machinery meticulously safeguards and transfers epigenetic information during cell division. Led by Professor Yuanliang Zhai at the School of Biological Sciences, The University of Hong Kong (HKU), alongside Professors Ning Gao and Qing Li from Peking University (PKU), and Professor Bik-Kwoon Tye from Cornell University, this collaborative endeavor holds promise for advancing treatments targeting specific gene activity changes in diseases like cancer, propelled by a deeper understanding of epigenetic mechanisms.
Within our bodies, various cell types thrive, each with its specialized functions, all orchestrated by genetic information encoded in our DNA. Despite containing identical DNA, different cells express distinct sets of genes, sculpting their unique identities and functions. This phenomenon, influenced by epigenetics, involves molecular switches dictating gene activation or silencing without altering the DNA sequence. Environmental factors such as diet, stress, and lifestyle mold these epigenetic switches, contributing to individual differences and disease susceptibilities, even among genetically identical individuals like identical twins.
Epigenetic processes unfold within the chromatin, where DNA wraps around histone proteins to form nucleosomes, the building blocks of chromatin. During DNA replication, these nucleosomes carrying epigenetic marks or histone modifications are dismantled and recycled. This ensures the faithful transfer of epigenetic information to newly forming cells, a crucial process for maintaining gene expression patterns and cell identity. Errors in this delicate choreography can disrupt epigenetic landscapes, altering gene expression profiles with implications for diseases like cancer and aging.
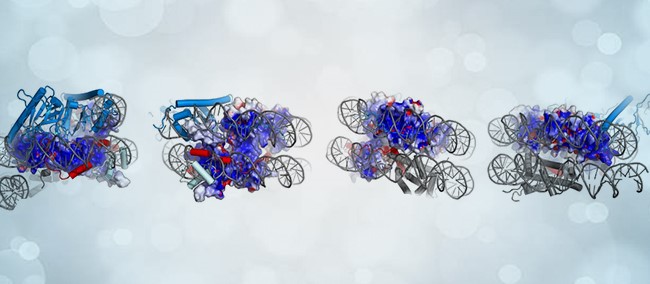
Previous studies by the research team laid the groundwork in understanding DNA replication mechanisms, setting the stage for their latest breakthrough. Leveraging cryo-electron microscopy (cryo-EM), they captured a pivotal moment in the transfer of parental histones at the replication fork, a crucial step in epigenetic inheritance.
Their investigation unveiled that the chaperone complex FACT, comprising Spt16 and Pob3, engages with parental histones ahead of the replication machinery. Spt16, a key component of FACT, seizes histones stripped from the parental nucleosome, forming a hexamer structure with one H2A-H2B dimer absent. Interestingly, Mcm2, a protein involved in DNA replication, fills the void left by the missing dimer, positioning the FACT-histone complex at the forefront of the replication engine, Tof1. This orchestrated interaction facilitates the transfer of parental histones onto newly synthesized DNA strands, ensuring the faithful propagation of epigenetic information.
Professor Zhai expressed his excitement over the findings, saying, “’It only took us less than four months from submission to Nature magazine to the acceptance of our manuscript. The results are incredibly beautiful. Our cryo-EM structures offer the first visual glimpse into how the DNA copying machine and FACT collaborate to transfer parental histone at the replication fork during DNA replication.” Although there is still much to be learned, Zhai and his team believe that each new discovery in this area will bring them closer to understanding epigenetic inheritance.
Beyond unraveling the intricacies of epigenetic inheritance, this breakthrough paves the way for deeper explorations into gene regulation, development, and disease. Furthermore, it offers tantalizing prospects for targeted therapies and innovative approaches to modulate epigenetic modifications, particularly in cancer treatment.
As the scientific community probes deeper into the realm of epigenetics, each stride forward, like this study, promises to unravel more layers of complexity, offering new avenues for therapeutic interventions and a deeper understanding of biological inheritance.
Source: Ningning Li et. al.Parental histone transfer caught at the replication fork. Nature, March 2024
Resource: The University of Hong Kong. Cracking Epigenetic Inheritance: HKU Biologists Discovered the Secrets of How Gene Traits are Passed on. March 7, 2024.

