A breakthrough genome-editing technique known as CRISPR has been shown by MIT biological engineers to disrupt a single parasite gene in a matter of weeks, with a success rate as great as 100 percent. In light of this and other recent research, the possibilities for the CRISPR/Cas9 system are substantial for precisely editing the genome and epigenome. With this new approach quicker gene analysis and bolstered drug-development efforts can occur, according to associate professor of biological engineering at MIT, Jacquin Niles.
One of the parasites that cause malaria, Plasmodium falciparum, has been incredibly resistant to researchers’ attempts to study its genetic makeup. Efforts to design targeted vaccines and drugs have proven notoriously difficult, especially when determining the function of just one of the parasite’s genes can take as long as a year.
“Even though we’ve sequenced the entire genome of Plasmodium falciparum, half of it still remains functionally uncharacterized. That’s about 2,500 genes that if only we knew what they did, we could think about novel therapeutics, whether it’s drugs or vaccines,” explains Niles, the senior author of a paper that describes the tool in an online edition of Nature Methods, published on Aug 10.
Jeffrey Wagner, the current study’s lead author, is a recent PhD recipient and currently a postdoc in biological engineering at MIT. Other contributors to the study include graduate student Randall Platt, recent PhD recipient Stephen Goldfless, and Feng Zhang, the W.M. Keck Career Development Assistant Professor in Biomedical Engineering.
The parasite, which resides in the blood and is carried by mosquitos, is the cause of a majority of the 655,000 deaths and 219 million estimated malaria cases per year. Artemisin and chloroquine are drugs currently used as malaria treatments, but P. falciparum is growing increasingly resistant.
New drugs must be developed, but possible genetic targets are difficult to identify. When conducting mice or other animal studies, it is common to assess gene functions by replacing a target gene with an artificial section of DNA or deleting the target gene. Applying this approach to P. falciparum can take as long as a year due to its reliance on homologous recombination. Homologous recombination is a form of genetic swapping utilized by cells to mend broken strands of DNA and it occurs rarely in the malaria parasite’s genome.
“You have to rely on this really inefficient process that occurs only if you have spontaneous DNA strand breaks that happen to fall within your region of interest,” says Niles.
With this slow and arduous method, researchers have successfully identified functions for certain genes necessary for the parasite’s invasion into and eruption from red blood cells. Researchers have also successfully snipped specific genes by using another approach that utilizes enzymes known as zinc finger nucleases. However, the zinc finger approach is expensive because a new nuclease must be designed for every gene target.
The revolutionary system for gene-editing called CRISPR has been created within the past couple years to exploit certain bacterial proteins that defend microbes against viral infection. The CRISPR system includes Cas9, an enzyme that cuts DNA. In this system, Cas9 is connected to a short RNA guide (gRNA) strand sequence that is programmed to bind to a particular genome sequence and indicate to Cas9 where to cut the DNA. Using this approach, scientists can target and remove any gene simply by altering the RNA guide strand sequence.
The CRISPR/Cas9 system could significantly influence medicine by helping to generate animal models and cell lines for therapeutic screening and evaluation, and also presents a new direction for gene therapy, according to three researchers who reviewed the developing technology in Cell Science & Report. The CRISPR/Cas9 system is especially effective in overcoming some limitations of other genetic and epigenetic editing techniques, such as a Zinc finger system and TALE system.
In terms of using the CRISPR/Cas9 system and the CRISPR/Cas9 antibody for epigenetic editing, the possibilities for innovative and ground-breaking research are vast. Using this system, histone or DNA modifying enzymes can fuse to a catalytically inactive Cas9 (dCas9) which can still be recruited by gRNAs without cleaving the DNA. This allows for the modulation of epigenetic states – such as DNA methylation or histone modification – of specific DNA sites.
Once researchers were able to demonstrate the success of this system in other cells as well as bacteria, Niles wondered whether he could manipulate specific genes of the malaria parasite Plasmodium falciparum. He and his colleagues tested this idea by using CRISPR to disturb two genes – eba-175 and kahrp – that had been successfully knocked out in malaria by using traditional techniques.
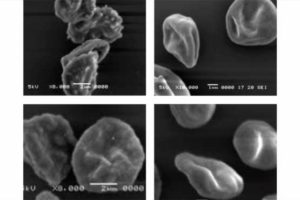
Red blood cells are normally smooth but for those infected with malaria, the kahrp gene creates a protein that makes the red blood cells knobby. After Niles’ team treated the parasites with CRISPR, they found that the gene was disrupted in 100 percent of the P. falciparum. And when red blood cells were infected by those mutant parasites, they remained smooth.
The gene eba-175 codes for a protein that attaches to red blood cell receptors and assists the parasite in entering the cells. When they treated the parasites with the CRISPR system, the researchers found that the gene was disrupted in 50 to 80 percent of the P. falciparum. “We consider this to be a win,” Niles says. “Compared to the efficiency with which P. falciparum genetics have been done in the past, even 50 percent is pretty substantial.”
The researchers also showed that they were able to insert a gene that codes for the light-emitting protein luciferase, as well as inactivate eba-175 and kahrp genes.
“The general concept of using the CRISPR/Cas9 system to edit the genome of the malaria parasite is significant because we’ve struggled with the technical aspects of doing these genetic experiments,” says Kirk Deitsch, a professor of microbiology and immunology at Cornell University who did not contribute to the study. “Now based on CRISPR, we can modify genes in a shorter timeframe and with greatly enhanced precision.”
Niles anticipates that numerous scientists will adopt the CRISPR technology for genetic studies of the parasite. Additional research could illuminate the process by which the parasite invades and replicates within blood cells, potentially leading to new vaccine and drug targets.
The CRISPR/Cas9 system opens new doors, not only for genetic editing, but also for epigenetic editing, enabling researchers to precisely alter methylation sites on a specific loci of interest and even influence chromatin states and histone modifications.
“I think the impact could be quite huge,” Niles says. “It lowers the barrier to really being more imaginative in terms of how we do experiments and the kinds of questions that we can ask.”
Source: Learn all about it and read more about their findings here: Jeffrey C Wagner, Randall J Platt, Stephen J Goldfless, Feng Zhang, Jacquin C Niles. Efficient CRISPR-Cas9–mediated genome editing in Plasmodium falciparum. Nature Methods. 2014.
References: MIT News. An easier way to manipulate malaria genes. 2014.
Zhao Y, Ying Y, Wang Y. Developing CRISPR/Cas9 Technologies for Research and Medicine. Cell Science Report. 2014.


