
Long-term exposure to cold temperatures could actually affect our gene expression and influence our fat cells, a recent study suggests. Research published in Nature Communications offers more evidence on how lifestyle choices and exposure to our environment can dictate the way our genes express themselves, an overarching theme of epigenetics.
A group of researchers based out of Tokyo worked alongside colleagues from around the world to track changes to the epigenome following long-term exposure to cold temperatures. They discovered that these epigenetic adjustments led white fat cells, which typically store energy, to turn into healthier “beige” fat cells that produce heat.
“We believe that this is the first time that anyone has collected data to prove that there are two steps between the environmental stimuli and epigenetic changes,” said Juro Sakai, PhD, a professor at the University of Tokyo and Tohoku University.
Epigenetics regulates gene expression via chemical tags or patterns that lie above the DNA sequence. Our genotype, or genetic makeup, is decided when we’re conceived, but the way we interact with our environment and the lifestyle choices we make can alter the epigenetic sequence over time, dynamically changing the way in which our genes are expressed. The most popular epigenetic mechanisms include DNA methylation and histone modifications.
Many scientists believe that there might be a stepwise process within the cell that manages environmental influences on the epigenome. According to the researchers, no specific molecular mechanisms of this process had been identified prior to their study.
“Understanding how the environment influences metabolism is scientifically, pharmacologically, and medically interesting,” Dr. Sakai said.
When we’re chilly, our body shivers in order to generate short-term heat by warming up the muscles. Shivering is a mechanical reflex we involuntarily engage in to maintain homeostasis. Thermogenesis, on the other hand, is a chemical process by which brown fat cells use lipids to create heat and keep the body warm for the long term. Brown fat is thought to be healthier and is not linked with metabolic diseases related to excess white fat.
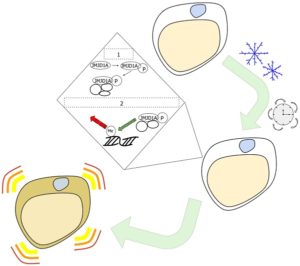
An organism’s sympathetic nervous system responds when it’s cold for a long time by releasing adrenaline, eventually reaching white fat if the cold persists. First, the cell begins to change an amino acid in a protein called JMJD1A, which is a histone H3 lysine 9 (H3K9) demethylase. The altered JMJD1A (also known as JHDM2A or KDM3A) then recruits other proteins.
Afterward, the JMJD1A protein complex is recruited to genes that begin thermogenesis. JMJD1A adjusts the epigenetic pattern to turn genes on, making them active. These particular epigenetic alterations transform white fat cells into beige fat cells, which, like brown fat cells, can carry out thermogenesis.
Specifically, the browning of white fat cells “occurs through a two-step process that requires both β-adrenergic-dependent phosphorylation of S265 and demethylation of H3K9me2 by JMJD1A,” the researchers reported.
Having fewer white fat cells and more beige fat cells may lessen the symptoms or negative health consequences of metabolic diseases, including obesity and diabetes. Even though turning white fat cells into beige and boosting thermogenesis is a natural stress response to chronic cold exposure, there are other ways the transition can occur without stress from the cold or adrenaline.
“Our next experiments will look more closely at epigenetic modifications within the thermogenesis signaling pathway so that we may manipulate it,” commented Sakai.
Many drugs for metabolic diseases currently depend on hormones that are systemic throughout the body. They may also focus on entire proteins. However, Sakai and her research team envision a time when single amino acids could be targeted to treat metabolic diseases.
“S265 phosphorylation of JMJD1A, and the following demethylation of H3K9me2 might prove to be a novel molecular target for the treatment of metabolic disorders, via promoting beige adipogenesis,” the research team wrote.
The JMJD1A protein is linked to numerous processes aside from thermogenesis, including infertility, cancer, determining the sex of an embryo, and the renewal of stem cells. Sakai and her group of researchers found sites within the protein sequence that are specific for controlling various activities of the protein. Targets for precision drugs could be revealed in the future by manipulating the specific amino acids.
Source: Abe, Y. et al. (2018). Histone demethylase JMJD1A coordinates acute and chronic adaptation to cold stress via thermogenic phospho-switch. Nature Communications, 9:1566.
Reference: University of Tokyo. Enduring cold temperatures alters fat cell epigenetics. Research Center for Advanced Science and Technology. 19 Apr 2018. Web.


